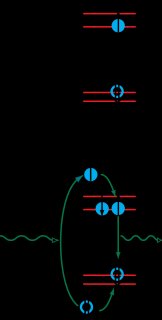
 Quantum dots (QDs) are often called “artificial atoms” because they locally confine single charges in discrete energy states analogous to the orbital energy levels of natural atoms. These artificial atoms are used in a variety of optoelectonic devices, including lasers, single photon sources, and optical and infrared detectors. QDs are also widely used in the biological sciences as fluorescent markers that enable the spatially resolved detection of target biomolecules. The size of the quantum dot, along with the material composition of the quantum dot and the confining barrier, determine the energy difference between allowed states of the conduction and valence band, as shown in the figure on the left. The ability to tune the energy levels is one of the features that make quantum dots very interesting for optoelectronic devices. One of the simplest optical properties is photoluminescence, which is schematically depicted in the bottom panel of the figure on the left. (1) A photon promotes an electron from the valence band to the conduction band, leaving behind a hole. (2) The electron and hole relax to the lowest allowed energy levels of the QD. (3) The electron and hole recombine to emit a lower-energy photon.
Quantum dots (QDs) are often called “artificial atoms” because they locally confine single charges in discrete energy states analogous to the orbital energy levels of natural atoms. These artificial atoms are used in a variety of optoelectonic devices, including lasers, single photon sources, and optical and infrared detectors. QDs are also widely used in the biological sciences as fluorescent markers that enable the spatially resolved detection of target biomolecules. The size of the quantum dot, along with the material composition of the quantum dot and the confining barrier, determine the energy difference between allowed states of the conduction and valence band, as shown in the figure on the left. The ability to tune the energy levels is one of the features that make quantum dots very interesting for optoelectronic devices. One of the simplest optical properties is photoluminescence, which is schematically depicted in the bottom panel of the figure on the left. (1) A photon promotes an electron from the valence band to the conduction band, leaving behind a hole. (2) The electron and hole relax to the lowest allowed energy levels of the QD. (3) The electron and hole recombine to emit a lower-energy photon. and device opportunities.
and device opportunities.
Recent advances in materials science and nanofabrication techniques have made it possible to controllably couple individual QDs to create “artificial molecules.” In natural molecules, the degree of quantum coupling is determined by the electronegativity of each atom and the equilibrium spacing between the atomic nuclei. In artificial molecules constructed of QDs the degree of quantum coupling can be engineered using precise control over the spatial positions and relative bandgaps of each QD. This control over quantum mechanical coupling at the level of single electrons and holes enables the design of novel materials with revolutionary properties.