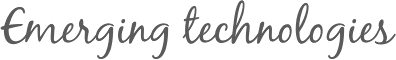 The scanning electron microscopy images of TZO products in Fig. 2a reveal that TiO2 NPs with diameters in the range of 20–250 nm are deposited on one of the frontier ends of ZnO NRs. These ZnO NRs have diameters in the range of 10–200 nm and lengths in the range of 1–3 μm. Fig. 2 shows that the typical morphology of a single TZO nanohybrid containing a cap-like TiO2 NP which covers one end of the ZnO NR. The ZnO NRs are well crystallized with no detected impurities in the EDX results. The titanium oxide particles assembled with NRs are identified as amorphous, and the stoichiometric ratio of Ti to O is approximately 1:2 as measured by EDS (see Fig. 2b).
The scanning electron microscopy images of TZO products in Fig. 2a reveal that TiO2 NPs with diameters in the range of 20–250 nm are deposited on one of the frontier ends of ZnO NRs. These ZnO NRs have diameters in the range of 10–200 nm and lengths in the range of 1–3 μm. Fig. 2 shows that the typical morphology of a single TZO nanohybrid containing a cap-like TiO2 NP which covers one end of the ZnO NR. The ZnO NRs are well crystallized with no detected impurities in the EDX results. The titanium oxide particles assembled with NRs are identified as amorphous, and the stoichiometric ratio of Ti to O is approximately 1:2 as measured by EDS (see Fig. 2b). ectron beam is perpendicular to the NRs axes. After annealing, the products are still well dispersed and the morphologies do not change (Fig. 2c and d) while the amorphous TiO2 caps have transformed to the anatase and rutile phases. The interface structures of almost all of the nanohybrids are similar and atomically flat. Readers can refer to our previous work, for more details about the formation mechanism of TZO and related structural characterizations.
ectron beam is perpendicular to the NRs axes. After annealing, the products are still well dispersed and the morphologies do not change (Fig. 2c and d) while the amorphous TiO2 caps have transformed to the anatase and rutile phases. The interface structures of almost all of the nanohybrids are similar and atomically flat. Readers can refer to our previous work, for more details about the formation mechanism of TZO and related structural characterizations.
(a) SEM image of TZO, (b) TEM and HRTEM images of a TZO and its EDS spectra, (c) and (d) SEM images of TZO300 and TZO600, (e) and (f) HRTEM images of TZO300 and TZO600, displaying the fine structures of the interface.
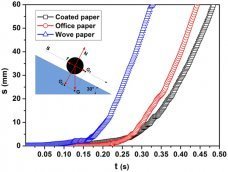
The UV-Vis absorption spectra of amorphous TiO2 NPs, ZnO NRs, TZO nanohybrids and the annealed products at 300°C (TZO300) and 600°C (TZO600) are compared in Fig. 3. ZnO NRs and all TZOs have strong adsorption in the UV region at the absorption edge of ca. 380 nm, with a corresponding ZnO bandgap of 3.26 eV. The amorphous TiO2 NPs have a strong adsorption in the deep-UV region at the absorption edge ca. 313 nm with a corresponding bandgap of 3.96 eV. For TZO, a higher absorbance below 320 nm ensures that the TiO2 NPs, which are attached to ZnO NRs, dominate the deep UV absorption. This may enhance the use of UV light compared with pure ZnO NRs.
Figure 3: UV-Vis absorption spectra of amorphous TiO2 NPs, ZnO NRs, TZO nanohybrids and the annealed products TZO300 and TZO600.