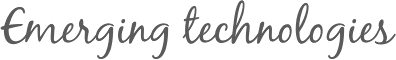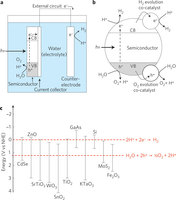
- Figure 2: Semiconductor photocatalysis.
a, Photoelectrochemical cell design for water splitting (processes for an n-type semiconductor are shown). When illuminated with photons (hν) of energy exceeding the bandgap, excited charge carriers are formed in the semiconductor photoanode. The holes diffuse to the semiconductor surface and drive the oxygen-evolution half-reaction (2H2O + 4h+ O2 + 4H+). Electrons are collected and travel to the counter electrode where they drive the hydrogen-evolution half-reaction (2H+ + 2e− H2). b, Particle-based water-splitting photocatalyst. Excited charge carriers (both electrons and holes) diffuse to the particle surface where they drive the two half-reactions, usually at specially designed co-catalyst sites. VB, valence band; CB, conduction band. c, VB and CB for a range of semiconductors (data from refs 90 and 91) on a potential scale (V) versus the normal hydrogen electrode (NHE). Redox potentials for the water-splitting half-reactions versus the NHE are also indicated by dashed red lines. For the water-splitting reaction to be thermodynamically favourable, the bandgap should straddle these redox potentials, that is, the CB should have higher energy (more negative potential) than the hydrogen-evolution potential and the VB should be lower in energy than the oxygen-evolution potential.
Nano-Structures for Optics and Photonics: Optical Strategies for Enhancing Sensing, Imaging, Communication and Energy Conversion (NATO Science for Peace and Security Series B: Physics and Biophysics)
Book (Springer)
|
Language clarification (to me) request
by gerundergrund[quote]
i'll give you +1 for sort of funny < - > 06/26 22:46:47
invisibility cloak via metamaterials, negative index materials, and plasmonic nanostructures...ok then
spare me the details
def regurgs please? :
"invisibility via metamaterials" >>>cheeky designatory reference terminology selection, because what's interdicted is in classified genre- wise as "metamaterial"--the light photons--non material so meta material. Like saying "I'm shooting bullets at him" and not "I'm shooting my gun at him". That's my understanding
Nanotubes Increase Solar PV Conductivity 100 Million-Fold — Sourceable
Carbon-based nanostructures are already being used as materials in solar cells with increasing frequency, yet their ability to enhance electrical performance has thus far been hampered by limited ability to assemble orderly networks using the materials.

|
Tutorials in Metamaterials (Series in Nano-Optics and Nanophotonics)
Book (CRC Press)
|
|
|
Nanophotonic Structures and Materials (A Wiley-Science Wise Co-Publication)
Book (Wiley)
|

|
Nanofabrication Handbook
Book (CRC Press)
|

|
Nanodroplets (Lecture Notes in Nanoscale Science and Technology)
eBooks (Springer)
|