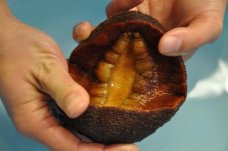
 A recent paper published in the journal Advanced Functional Materials, reveals how the largest type of chitons- gumboot chitons, can contribute to improving solar cells and lithium-ion batteries.
A recent paper published in the journal Advanced Functional Materials, reveals how the largest type of chitons- gumboot chitons, can contribute to improving solar cells and lithium-ion batteries.
Professor David Kisailus, from the University of California, Riverside’s Bourns College of Engineering, together with his team of students and scientists at Harvard University, University in Cambridge Mass., Chapman University in Orange, Calif. and Brookhaven National Laboratory in Upton, NY, designed the revolutionary engineering products and materials.
have been of interest to Kisailus for about five years. Initially he was interested in abrasion and impact-resistant materials. Some of his earlier findings indicate that chiton teeth contain magnetite- the hardest biomaterial known on Earth.
The professor used his previous knowledge and built on it to come up with his ground-breaking innovation. The aim of his work was to determine the exact process of formation of the magnetic region of the chiton’s teeth.
This research identified three crucial steps in the formation of the material. Firstly, hydrated iron oxide (ferrihydrite) crystals nucleate on a fiber-like chitinous (complex sugar) organic template. Through the process of solid-state transformation, ferrihydrite particles convert to a magnetic iron oxide (magnetite). The strength of the teeth comes from the yielding of parallel rods within the teeth, which occurs as a result of the organic fibers growing along the magnetite particles.
This process occurs at a room temperature, naturally bringing down the production cost of the nanomaterials. This is the procedure Kisailus follows in order to grow minerals used in solar cells and lithium-ion batteries under laboratory conditions.
He is convinced that building such materials is possible and they will help solar cells and lithium-ion batteries operate more efficiently.