 Carbon is a critical element to plant and animal life, while simultaneously found in many non-biological compounds as well. If we are thinking about the future, and thinking about the cutting-edge science being done today that will change our lives forever, we have to understand the role that carbon will play, as well as some of its fundamental characteristics. In short, we've got to talk "carbon"!
Carbon is a critical element to plant and animal life, while simultaneously found in many non-biological compounds as well. If we are thinking about the future, and thinking about the cutting-edge science being done today that will change our lives forever, we have to understand the role that carbon will play, as well as some of its fundamental characteristics. In short, we've got to talk "carbon"!
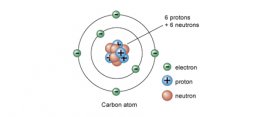
Taking a look at a Periodic Table, we can see that carbon is in Period (row) 2, and Group 14. umber of 6, as well as an atomic weight of 12.01 amu (atomic mass units). Our take away from that is as follows: because Carbon has an atomic number of 6, we know that it has 6 protons (6 positively charged subatomic particles), and if Carbon is in its neutral state, having a charge of 0 (a/k/a having no charge), it has 6 electrons (6 negatively charged subatomic particles) as well. Its atomic mass of 12.01 amu is the sum of the masses of all the protons, electrons and neutrons (subatomic particles with no charge) in a single Carbon atom.
umber of 6, as well as an atomic weight of 12.01 amu (atomic mass units). Our take away from that is as follows: because Carbon has an atomic number of 6, we know that it has 6 protons (6 positively charged subatomic particles), and if Carbon is in its neutral state, having a charge of 0 (a/k/a having no charge), it has 6 electrons (6 negatively charged subatomic particles) as well. Its atomic mass of 12.01 amu is the sum of the masses of all the protons, electrons and neutrons (subatomic particles with no charge) in a single Carbon atom.
Now, lets talk about bonding. A chemical bond (H 2 O, CH2O - formaldehyde, and C6H12O6, glucose) is created when two atoms decide to share electrons with each other. In other words, in water, Oxygen shares one electron with one Hydrogen and another electron with another Hydrogen, creating H2O. The electrons they are sharing are called valence electrons. Valence electrons are electrons that exist in the outer shell of atoms, as those are the only electrons that can be shared. There is much more to say on the electronic structure of a given atom, but for our purposes, let's stick with the concept of one valence electron of one atom bonding with one valence electron of another atom to create a bond.