 † Chemical Sciences and Material Sciences Divisions, Lawrence Berkeley National Laboratory, Berkeley, California 94720, United States
† Chemical Sciences and Material Sciences Divisions, Lawrence Berkeley National Laboratory, Berkeley, California 94720, United States
‡ Department of Chemistry, University of California, Berkeley, California 94720, United States
§ Department of Mechanical Engineering, University of California, Berkeley, California 94720, United States
J. Phys. Chem. C, 2013, 117 (4), pp 1809–1817
DOI: 10.1021/jp311772p
Section:
Abstract
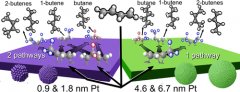
The product selectivity during 1, 3-butadiene hydrogenation on monodisperse, colloidally synthesized, Pt nanoparticles was studied under reaction conditions with kinetic measurements and in situ sum frequency generation (SFG) vibrational spectroscopy. SFG was performed with the capping ligands intact in order to maintain nanoparticle size by reduced sintering. Four products are formed at 75 °C: 1-butene, cis-2-butene, trans-2-butene, and n-butane. Ensembles of Pt nanoparticles with average diameters of 0.9 and 1.8 nm exhibit a 30% and 20% increase in the full hydrogenation products, respectively, as compared to Pt nanoparticles with average diameters of 4.6 and 6.7 nm. Methyl and methylene vibrational stretches of reaction intermediates observed under working conditions using SFG were used to correlate the stable reaction intermediates with the product distribution. Kinetic and SFG results correlate with previous DFT predictions for two parallel reaction pathways of 1, 3-butadiene hydrogenation. at internal or terminal carbons leading to the formation of 1-buten-4-yl radical (metallocycle) and 2-buten-1-yl radical intermediates, respectively. Small (0.9 and 1.8 nm) nanoparticles exhibited vibrational resonances originating from both intermediates, while the large (4.6 and 6.7 nm) particles exhibited vibrational resonances originating predominately from the 2-buten-1-yl radical. This suggests each reaction pathway competes for partial and full hydrogenation and the nanoparticle size affects the kinetic preference for the two pathways. The reaction pathway through the metallocycle intermediate on the small nanoparticles is likely due to the presence of low-coordinated sites.
at internal or terminal carbons leading to the formation of 1-buten-4-yl radical (metallocycle) and 2-buten-1-yl radical intermediates, respectively. Small (0.9 and 1.8 nm) nanoparticles exhibited vibrational resonances originating from both intermediates, while the large (4.6 and 6.7 nm) particles exhibited vibrational resonances originating predominately from the 2-buten-1-yl radical. This suggests each reaction pathway competes for partial and full hydrogenation and the nanoparticle size affects the kinetic preference for the two pathways. The reaction pathway through the metallocycle intermediate on the small nanoparticles is likely due to the presence of low-coordinated sites.
Citing Articles
Citation data is made available by participants in CrossRef's Cited-by Linking service. For a more comprehensive list of citations to this article, users are encouraged to perform a search in SciFinder.
 Because I believe
Because I believe  One who loves God will see what they believe and the ones who trust science will believe what they see
One who loves God will see what they believe and the ones who trust science will believe what they see




