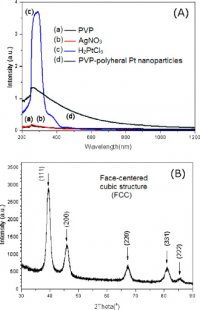
2. Experimental
The chemicals used in our process were bought from Aldrich and Sigma-Aldrich. They were polyvinylpyrrolidone with the chemical formula (C6H9NO) (PVP, MW=55 000) used as a stabilizer, and chloroplantinic acid hexahydrate as an important compound (H2PtCl66H2O, ACS reagent) used as a good precursor for Pt nanoparticles. Ethylene glycol (EG) (C2H6O2, 95.5%) was used as both the solvent and the reducing agent. Silver nitrate (AgNO3, metal basis, 99.9999%) was used as a structure-modifying agent. In a typical process, chemicals used are poly-vinylpyrrolidone (PVP), chloroplantinic acid hexahydrate H2PtCl66H2O, ethylene glycol (EG), silver nitrate AgNO3, metals basis, ethanol, acetone and hexane, ionized and distilled water by Narnstead nanopure H2O purification system. We used the stock solutions of 3 ml EG and 0.5 ml 0.04 M AgNO3, 2 ml 0.0625 M H2PtCl6, and 4 ml 0.375 M PVP. First, a small volume of 0.0625 M H2PtCl6 of total volume of 2 ml, and a small volume of PVP of 0.375 M, are simultaneously added into the volumetric flask many times (every 60 s or 30 s for a pump), so that a volume of the solution of 0.375 M PVP was more than twice the volume of the solution of 0.0625 M H2PtCl6 (20 μl of H2PtCl6 and 40 μl of PVP via a syringe) until 2 ml of H2PtCl6 and 4 ml of 0.375 M PVP were thoroughly used. The reduction of H2PtCl6 by EG occurring and finishing within 10–30 min was necessary for achieving their sharply polyhedral morphologies. The resultant mixture was heated and refluxed at 160 °C for the chemical reduction of H2PtCl6, and the color of the resultant solution became dark-brown. For the polyhedral morphologies, the reduction of H2PtCl6 by EG occurred and was finished within 10–30 min. In contrast, the reduction of H2PtCl6 by EG occurred for 15 min, and the product was kept in the flask for 6 h to obtain non-sharp and non-polyhedral shapes [, ]. The fixed volumes were collected for the UV-Vis-near infrared (NIR) measurements. To obtain the fresh Pt nanoparticles without PVP polymer for various measurements, the product was centrifuged at 15 000 rpm for 15 min using a Sigma 3K30C-Kubota centrifuge. The supernatant was separated and precipitated by adding a triple volume of acetone to remove any impurities from outside. We continued to carry out the centrifugation at 12 000 rpm for 30 min again. The precipitate was collected and diluted in 2 ml of ethanol with sonication for 15 min to generate Pt colloidal solution by an ultrasound generator (200 W/37 kHz), and release them randomly in ethanol. Next, 6 ml volume of hexane was added to make the dispersion adequately, and the solution was centrifuged at 3000 rpm for 10 min. To obtain the pure nanoparticles for experimental measurements, the precipitate was washed several times with the same mixture of ethanol/hexane to remove the remaining impurities. After that, the precipitate of Pt nanoparticles was dispersed in 3 ml of ethanol or milli-Q water. Then, the as-prepared Pt nanoparticles were used to study the electroactivity of catalyst electrodes [, ]. The TEM and scanning TEM (STEM) images of the pure Pt nanoparticles were obtained using a transmission electron microscopy (TEM) (JEOL JEM-2100F and JEM-2010) operated at 200 kV.
 Because I believe
Because I believe  One who loves God will see what they believe and the ones who trust science will believe what they see
One who loves God will see what they believe and the ones who trust science will believe what they see




