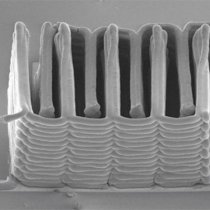
 This image shows the interlaced stack of electrodes that were printed layer by layer to create the working anode and cathode of a microbattery. (SEM image courtesy of Jennifer A. Lewis.)
This image shows the interlaced stack of electrodes that were printed layer by layer to create the working anode and cathode of a microbattery. (SEM image courtesy of Jennifer A. Lewis.)
Materials scientists at Harvard University have been recognized by the printed electronics industry for their work on novel 3D-printed lithium-ion microbatteries the size of a grain of sand.
The printed microbatteries, which could supply electricity to tiny devices in fields from medicine to communications, received the Academic R&D Award on November 20 at the IDTechEx Printed Electronics USA 2013 event in Santa Clara, California.
The award, recognizing "innovation, success and development, " was presented to Jennifer A. Lewis, Hansjörg Wyss Professor of Biologically Inspired Engineering at Harvard School of Engineering and Applied Sciences (SEAS), and a Core Faculty Member of the Wyss Institute for Biologically Inspired Engineering at Harvard University.
The Academic category recognizes researchers who have "made a significant contribution over the past 24 months to the understanding of the principles and accrued knowledge behind printed electronics."
According to the award description, Lewis' research team at Harvard "has printed rechargeable 3D Li-ion microbatteries composed of high–aspect ratio anode and cathode micro-arrays that are interdigitated on a sub-millimeter scale, and exhibit amongst the highest area energy and power densities reported to date. These microbatteries occupy volumes less than 1 mm3—equivalent in size to a single grain of sand—and are 1000 times smaller than the smallest commercially available rechargeable batteries. The university is working on developing these batteries for a broad range of autonomous applications, including biomedical devices, micro-UAVs, and distributed sensor arrays (e.g., smart dust)."
 The work was announced in June 2013 and published online in the journal Advanced Materials.
The work was announced in June 2013 and published online in the journal Advanced Materials.
Lewis and co-author Shen Dillon, Assistant Professor of Materials Science and Engineering at the University of Illinois at Urbana-Champaign, collaborated with lead author Ke Sun, a graduate student in Materials Science and Engineering at the University of Illinois at Urbana-Champaign; Teng-Sing Wei, a graduate student at Harvard SEAS; Bok Yeop Ahn, a Senior Research Scientist at the Wyss Institute and SEAS; and Jung Yoon Seo, a visiting scientist in the Lewis group, from the Korea Advanced Institute of Science and Technology.